SAN MATEO, Calif. - Wednesday, 07. August 2024 AETOSWire
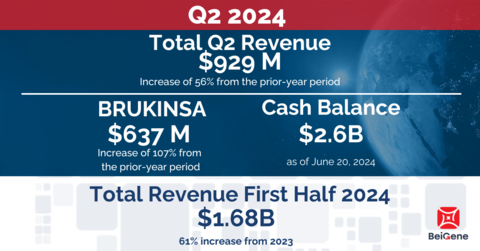
Generated total revenues of $929 million, an increase of 56% from the
prior-year period; reduced GAAP operating loss and achieved non-GAAP
operating income
Strengthened hematology leadership with global
BRUKINSA revenues of $637 million, an increase of 107% from the
prior-year period; advanced pivotal programs for BCL2 inhibitor
sonrotoclax and BTK-targeted degrader BGB-16673
Advanced
innovative solid tumor pipeline of more than 15 investigational
molecules, including ADCs, multispecific antibodies, and targeted
therapies for lung, breast, and gastrointestinal cancers
Strengthened global presence with opening of $800 million, 42-acre
flagship U.S. biologics manufacturing facility and clinical R&D
center in New Jersey and proposal to redomicile from Cayman Islands to
Switzerland, an innovative biotech ecosystem for life sciences leaders
and institutions
(BUSINESS WIRE) -- BeiGene, Ltd.
(NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global oncology company,
today announced results from the second quarter 2024 and corporate
updates that strengthen the Company for future global growth.
“This
was a tremendous second quarter and an inflection point as BeiGene
achieved positive non-GAAP operating income with rapidly increasing
global revenues and continued financial discipline. Having now reached
this milestone, we will further build on our differentiated, strategic
capabilities as a leading, global oncology innovator,” said John V.
Oyler, Co-Founder, Chairman and CEO of BeiGene. “BRUKINSA is emerging as
the BTKi class leader in the U.S. in new patient starts across all
approved indications, demonstrating the strength of its clinical
efficacy and safety data, and is the only BTKi to demonstrate superior
efficacy versus ibrutinib in a head-to-head trial. With our leadership
in hematology, we are working to expand into other highly prevalent
cancer types, backed by one of the largest oncology research teams in
the industry. With our continued growth in established biopharmaceutical
hubs such as New Jersey and Switzerland, we are better positioned to
reach even more patients with our innovative medicines.”
Financial Highlights
(Amounts in thousands of U.S. dollars)
Three Months Ended June 30,
Six Months Ended June 30,
(in thousands, except percentages)
2024
2023
% Change
2024
2023
% Change
Net product revenues
$
921,146
$
553,745
66
%
$
1,668,064
$
964,036
73
%
Net revenue from collaborations
$
8,020
$
41,516
(81
)%
$
12,754
$
79,026
(84
)%
Total Revenue
$
929,166
$
595,261
56
%
$
1,680,818
$
1,043,062
61
%
GAAP loss from operations
$
(107,161
)
$
(318,715
)
(66
)%
$
(368,509
)
$
(689,973
)
(47
)%
Adjusted income(loss) from operations*
$
48,464
$
(193,051
)
125
%
$
(98,877
)
$
(468,910
)
(79
)%
*
For an explanation of our use of non-GAAP financial measures refer to
the "Use of Non-GAAP Financial Measures" section later in this press
release and for a reconciliation of each non-GAAP financial measure to
the most comparable GAAP measures, see the table at the end of this
press release.
Key Business Updates
BRUKINSA® (zanubrutinib)
U.S. sales of BRUKINSA totaled $479 million in the second quarter of
2024, representing growth of 114% over the prior-year period, with more
than 60% of the quarter over quarter demand growth coming from expanded
use in CLL as BRUKINSA continued to gain share in CLL new patient
starts; BRUKINSA sales in Europe totaled $81 million in the second
quarter of 2024, representing growth of 209%, driven by increased market
share across all major markets, including Germany, Italy, Spain, France
and the UK;
Presented data from Arm D of the Phase 3 SEQUOIA
trial evaluating BRUKINSA in combination with venetoclax in
treatment-naïve (TN) patients with high-risk CLL and/or small
lymphocytic lymphoma (SLL) with del(17p) and/or TP53 mutation as an oral
presentation at the European Hematology Association (EHA) 2024 Hybrid
Congress; preliminary data demonstrated an overall response rate of 100%
in 65 response-evaluable patients and a rate of complete response (CR)
plus CR with incomplete hematopoietic recovery (CRi) of 48%; and
Presented new analyses highlighting improved progression free survival
and response rates and a low usage of antihypertensive medicines for
patients treated with BRUKINSA compared to other Bruton’s tyrosine
kinase inhibitors (BTKis) used to treat CLL/SLL, including acalabrutinib
and ibrutinib at the American Society of Clinical Oncology (ASCO)
Annual Meeting and EHA.
TEVIMBRA® (tislelizumab)
Sales
of tislelizumab totaled $158 million in the second quarter of 2024,
representing growth of 6% compared to the prior-year period;
Presented new data from the Phase 3 RATIONALE-306 study evaluating
TEVIMBRA plus chemotherapy in patients with advanced or metastatic
esophageal squamous cell carcinoma (ESCC) at ASCO; and
Received an update that the U.S. Food and Drug Administration (FDA) has
deferred approval for tislelizumab in first-line unresectable,
recurrent, locally advanced, or metastatic ESCC with a target PDUFA
action date of July 2024 on account of a delay in scheduling clinical
site inspections.
Key Pipeline Highlights
Hematology
Sonrotoclax (BCL2 inhibitor)
More than 1,000 patients enrolled to date across the program;
Completed enrollment in global Phase 2 trial in R/R mantle cell
lymphoma (MCL) and continued enrollment in global Phase 2 trial in
Waldenström’s macroglobulinemia (WM) and China-only Phase 2 trial in R/R
CLL, all with registrational intent, as well as continued enrollment in
global Phase 3 CELESTIAL trial in combination with BRUKINSA in TN CLL;
At EHA 2024, presented data highlighting deep and durable responses
with tolerable safety profile in Phase 1 studies in combination with
BRUKINSA in R/R CLL/SLL and R/R MCL as well as results of additional
Phase 1 trials demonstrating encouraging response rates, durable
responses and manageable safety profiles as monotherapy in R/R WM, in
combination with azacitidine in both TN and R/R acute myeloid leukemia,
and in combination with dexamethasone in R/R multiple myeloma harboring
translocation (11;14);
Received FDA fast track designation for R/R WM; and
Anticipating first subjects enrolled in Phase 3 programs in R/R CLL and
R/R MCL in the fourth quarter of 2024 or first quarter of 2025.
BGB-16673 (BTK CDAC)
More than 300 patients enrolled to date across the program; continued
to enroll potentially registration enabling expansion cohorts in R/R MCL
and R/R CLL; and
At EHA 2024, presented data highlighting
promising preliminary efficacy and safety in patients with R/R CLL/SLL;
anticipating first subject enrolled in Phase 3 program in fourth quarter
of 2024 or first quarter of 2025.
Solid Tumors
Lung Cancer
Multiple randomized tislelizumab lung cancer combination cohorts with
BGB-A445 (anti-OX40), LBL-007 (anti-LAG3) and BGB-15025 (HPK1 inhibitor)
expected to read out in 2024;
BGB-C354 (B7H3 ADC): Initiated dose escalation for the Company’s first internally developed ADC;
BGB-R046 (IL-15 prodrug): Initiated dose escalation; this is a cytokine
prodrug, leveraging protease-dependent release of active IL-15 in the
tumor microenvironment and eliciting anti-tumor activity by promoting T
and natural killer (NK) cell expansion; and
Pan-KRAS,
MTA-cooperative PRMT5 inhibitors and EGFR CDAC targeted protein degrader
on track to enter the clinic in the second half of 2024.
Breast and Gynecologic Cancers
BGB-43395 (CDK4 inhibitor): Continued dose escalation in monotherapy
and in combination with fulvestrant and letrozole in the anticipated
efficacious dose range with no dose limiting toxicities observed; more
than 60 patients enrolled to date across the program; potential to share
first readout of Phase 1 data in the fourth quarter of 2024; and
BG-68501 (CDK2 inhibitor) and BG-C9074 (B7H4 ADC): Continued
monotherapy dose escalation, with pharmacokinetics as expected and no
dose limiting toxicities observed.
Gastrointestinal Cancers
Tislelizumab combination cohorts with LBL-007 (anti-LAG3) in ESCC reading out in 2024;
BLA accepted by the NMPA for zanidatamab for the treatment of second-line biliary tract cancer; and
CEA ADC, FGFR2b ADC and GPC3x4-1BB bispecific antibody on track to enter the clinic in the second half of 2024.
Immunology & Inflammation
Initiated clinical development of BGB-43035 (IRAK4 CDAC) with potential
to induce deeper and faster IRAK4 degradation with stronger cytokine
inhibition than competitors; this is the second targeted degrader from
the Company’s proprietary CDAC platform.
Corporate Updates
Opened flagship U.S. biologics manufacturing facility and clinical
R&D center at the Princeton West Innovation Campus in Hopewell,
N.J.; the facility includes 400,000 square feet of dedicated
manufacturing space; and
Announced intent to change
jurisdiction of incorporation from the Cayman Islands to Basel,
Switzerland, enabling the Company to deepen its roots in a global
biopharmaceutical hub as it further executes on its global growth
strategy to reach more patients around the world with its innovative
medicines; this redomiciliation is subject to shareholder approval.
Second Quarter 2024 Financial Highlights
Revenue
for the three months ended June 30, 2024, was $929 million, compared to
$595 million in the same period of 2023, driven primarily by growth in
BRUKINSA product sales in the U.S. and Europe of 114% and 209%
respectively.
Product Revenue for the three months ended June 30,
2024, was $921 million, compared to $554 million in the same period of
2023, representing an increase of 66%. The increase in product revenue
was primarily attributable to increased sales of BRUKINSA. For the three
months ended June 30, 2024, the U.S. was the Company’s largest market,
with product revenue of $479 million, compared to $224 million in the
prior year period. In addition to BRUKINSA revenue growth, product
revenues were positively impacted by sales of in-licensed products from
Amgen in China and tislelizumab.
Gross Margin as a percentage of
global product revenue for the second quarter of 2024 was 85%, compared
to 83% in the prior-year period. The gross margin percentage increased
primarily due to proportionally higher sales mix of global BRUKINSA
compared to other products in the portfolio.
Operating Expenses
The following table summarizes operating expenses for the second quarter 2024 and 2023, respectively:
GAAP
Non-GAAP
(in thousands, except percentages)
Q2 2024
Q2 2023
% Change
Q2 2024
Q2 2023
%
Research and development
$
454,466
$
422,764
7
%
$
382,509
$
363,735
5
%
Selling, general and administrative
$
443,729
$
395,034
12
%
$
363,922
$
331,607
10
%
Amortization
$
—
$
188
(100
)%
$
—
$
—
NM
Total operating expenses
$
898,195
$
817,986
10
%
$
746,431
$
695,342
7
%
The following table summarizes operating expenses for the first half 2024 and 2023, respectively:
GAAP
Non-GAAP
(in thousands, except percentages)
Q2 YTD 2024
Q2 YTD 2023
% Change
Q2 YTD 2024
Q2 YTD 2023
% Change
Research and development
$
915,104
$
831,348
10
%
$
787,949
$
725,431
9
%
Selling, general and administrative
$
871,156
$
723,533
20
%
$
736,068
$
614,761
20
%
Amortization
$
—
$
375
(100
)%
$
—
$
—
NM
Total operating expenses
$
1,786,260
$
1,555,256
15
%
$
1,524,017
$
1,340,192
14
%
Research
and Development (R&D) Expenses increased for the second quarter of
2024 compared to the prior-year period on both a GAAP and adjusted basis
primarily due to advancing preclinical programs into the clinic and
early clinical programs into late stage. Upfront fees and milestone
payments related to in-process R&D for in-licensed assets totaled
$12 million in the second quarter of 2024, compared to nil in the
prior-year period.
Selling, General and Administrative (SG&A)
Expenses increased for the second quarter of 2024 compared to the
prior-year period on both a GAAP and adjusted basis due to continued
investment in the global commercial launch of BRUKINSA, primarily in the
U.S. and Europe. SG&A expenses as a percentage of product sales
were 48% for the second quarter of 2024 compared to 71% in the prior
year period.
Income (Loss) from Operations in the second quarter
of 2024 operating loss decreased 66% on a GAAP basis. On an adjusted
basis, we achieved operating income of $48 million. The decrease in GAAP
operating loss and achievement of profitability on an adjusted basis is
a key strategic goal and the result of tremendous efforts to drive
growth while maintaining investment discipline.
GAAP Net Loss
improved for the quarter ended June 30, 2024, compared to the prior-year
period, as our product revenue growth and management of expenses is
driving increased operating leverage.
For the quarter ended June
30, 2024, net loss per share were $(0.09) and $(1.15) per American
Depositary Share (ADS), compared to $(0.28) per share and $(3.64) per
ADS in the prior year period.
Cash Used in Operations for the
quarter ended June 30, 2024, totaled $96 million compared to $294
million in the prior-year period, driven by improved operating leverage.
For
further details on BeiGene’s Second Quarter 2024 Financial Statements,
please see BeiGene’s Quarterly Report on Form 10-Q for the second
quarter of 2024 filed with the U.S. Securities and Exchange Commission.
About BeiGene
BeiGene
is a global oncology company that is discovering and developing
innovative treatments that are more affordable and accessible to cancer
patients worldwide. With a broad portfolio, we are expediting
development of our diverse pipeline of novel therapeutics through our
internal capabilities and collaborations. We are committed to radically
improving access to medicines for far more patients who need them. Our
growing global team of more than 10,000 colleagues spans five
continents. To learn more about BeiGene, please visit www.beigene.com
and follow us on LinkedIn, X (formerly known as Twitter) and Facebook.
Forward-Looking Statements
This
press release contains forward-looking statements within the meaning of
the Private Securities Litigation Reform Act of 1995 and other federal
securities laws, including statements regarding BeiGene’s potential to
further emerge as a leading, global oncology innovator; BeiGene’s
ability to expand into other highly prevalent cancer types; BeiGene’s
preliminary clinical data and activities, as well as anticipated read
outs; whether shareholders will approve BeiGene’s change in jurisdiction
of incorporation and if approved, whether this change will enable
BeiGene to further execute on its global growth strategy; and BeiGene’s
plans, commitments, aspirations and goals under the caption “About
BeiGene”. Actual results may differ materially from those indicated in
the forward-looking statements as a result of various important factors,
including BeiGene’s ability to demonstrate the efficacy and safety of
its drug candidates; the clinical results for its drug candidates, which
may not support further development or marketing approval; actions of
regulatory agencies, which may affect the initiation, timing and
progress of clinical trials and marketing approval; BeiGene’s ability to
achieve commercial success for its marketed medicines and drug
candidates, if approved; BeiGene's ability to obtain and maintain
protection of intellectual property for its medicines and technology;
BeiGene’s reliance on third parties to conduct drug development,
manufacturing, commercialization, and other services; BeiGene’s limited
experience in obtaining regulatory approvals and commercializing
pharmaceutical products; BeiGene’s ability to obtain additional funding
for operations and to complete the development of its drug candidates
and achieve and maintain profitability; and those risks more fully
discussed in the section entitled “Risk Factors” in BeiGene’s most
recent quarterly report on Form 10-Q, as well as discussions of
potential risks, uncertainties, and other important factors in BeiGene’s
subsequent filings with the U.S. Securities and Exchange Commission.
All information in this press release is as of the date of this press
release, and BeiGene undertakes no duty to update such information
unless required by law.
Condensed Consolidated Statements of Operations (U.S. GAAP)
(Amounts in thousands of U.S. dollars, except for shares, American Depositary Shares (ADSs), per share and per ADS data)
Three Months Ended
June 30,
Six Months Ended
June 30,
2024
2023
2024
2023
(Unaudited)
(Unaudited)
Revenues
Product revenue, net
$
921,146
$
553,745
$
1,668,064
$
964,036
Collaboration revenue
8,020
41,516
12,754
79,026
Total revenues
929,166
595,261
1,680,818
1,043,062
Cost of sales - products
138,132
95,990
263,067
177,779
Gross profit
791,034
499,271
1,417,751
865,283
Operating expenses:
Research and development
454,466
422,764
915,104
831,348
Selling, general and administrative
443,729
395,034
871,156
723,533
Amortization of intangible assets
—
188
—
375
Total operating expenses
898,195
817,986
1,786,260
1,555,256
Loss from operations
(107,161
)
(318,715
)
(368,509
)
(689,973
)
Interest income, net
13,225
15,070
29,385
31,086
Other expense, net
(11,984
)
(63,818
)
(10,222
)
(45,515
)
Loss before income taxes
(105,920
)
(367,463
)
(349,346
)
(704,402
)
Income tax expense
14,485
13,674
22,209
25,166
Net loss
(120,405
)
(381,137
)
(371,555
)
(729,568
)
Net loss per share, basic and diluted
$
(0.09
)
$
(0.28
)
$
(0.27
)
$
(0.54
)
Weighted-average shares outstanding—basic and diluted
1,361,082,567
1,360,224,377
1,358,315,145
1,357,211,308
Net loss per ADS, basic and diluted
$
(1.15
)
$
(3.64
)
$
(3.56
)
$
(6.99
)
Weighted-average ADSs outstanding—basic and diluted
104,698,659
104,632,644
104,485,780
104,400,870
Select Condensed Consolidated Balance Sheet Data (U.S. GAAP)
(Amounts in thousands of U.S. Dollars)
As of
June 30,
December 31,
2024
2023
(unaudited)
(audited)
Assets:
Cash, cash equivalents, and restricted cash
$
2,617,931
$
3,185,984
Accounts receivable, net
529,449
358,027
Inventories
443,260
416,122
Property, plant and equipment, net
1,516,491
1,324,154
Total assets
5,712,179
5,805,275
Liabilities and equity:
Accounts payable
333,022
315,111
Accrued expenses and other payables
646,538
693,731
R&D cost share liability
203,627
238,666
Debt
1,036,928
885,984
Total liabilities
2,345,924
2,267,948
Total equity
$
3,366,255
$
3,537,327
Note Regarding Use of Non-GAAP Financial Measures
BeiGene
provides certain non-GAAP financial measures, including Adjusted
Operating Expenses and Adjusted Operating Loss and certain other
non-GAAP income statement line items, each of which include adjustments
to GAAP figures. These non-GAAP financial measures are intended to
provide additional information on BeiGene’s operating performance.
Adjustments to BeiGene’s GAAP figures exclude, as applicable, non-cash
items such as share-based compensation, depreciation and amortization.
Certain other special items or substantive events may also be included
in the non-GAAP adjustments periodically when their magnitude is
significant within the periods incurred. BeiGene maintains an
established non-GAAP policy that guides the determination of what costs
will be excluded in non-GAAP financial measures and the related
protocols, controls and approval with respect to the use of such
measures. BeiGene believes that these non-GAAP financial measures, when
considered together with the GAAP figures, can enhance an overall
understanding of BeiGene’s operating performance. The non-GAAP financial
measures are included with the intent of providing investors with a
more complete understanding of the Company’s historical and expected
financial results and trends and to facilitate comparisons between
periods and with respect to projected information. In addition, these
non-GAAP financial measures are among the indicators BeiGene’s
management uses for planning and forecasting purposes and measuring the
Company’s performance. These non-GAAP financial measures should be
considered in addition to, and not as a substitute for, or superior to,
financial measures calculated in accordance with GAAP. The non-GAAP
financial measures used by the Company may be calculated differently
from, and therefore may not be comparable to, non-GAAP financial
measures used by other companies.
RECONCILIATION OF SELECTED GAAP MEASURES TO NON-GAAP MEASURES
(in thousands, except per share amounts)
(unaudited)
Three Months Ended
Six Months Ended
June 30,
June 30,
2024
2023
2024
2023
(in thousands)
(in thousands)
Reconciliation of GAAP to adjusted cost of sales - products:
GAAP cost of sales - products
$
138,132
$
95,990
$
263,067
$
177,779
Less: Depreciation
2,684
2,180
5,029
4,360
Less: Amortization of intangibles
1,177
840
2,360
1,639
Adjusted cost of sales - products
$
134,271
$
92,970
$
255,678
$
171,780
Reconciliation of GAAP to adjusted research and development:
GAAP research and development
$
454,466
$
422,764
$
915,104
$
831,348
Less: Share-based compensation cost
55,406
45,948
93,451
79,976
Less: Depreciation
16,551
13,081
33,704
25,941
Adjusted research and development
$
382,509
$
363,735
$
787,949
$
725,431
Reconciliation of GAAP to adjusted selling, general and administrative:
GAAP selling, general and administrative
$
443,729
$
395,034
$
871,156
$
723,533
Less: Share-based compensation cost
75,288
57,381
125,957
98,741
Less: Depreciation
4,519
6,046
9,131
10,031
Adjusted selling, general and administrative
$
363,922
$
331,607
$
736,068
$
614,761
Reconciliation of GAAP to adjusted operating expenses
GAAP operating expenses
$
898,195
$
817,986
$
1,786,260
$
1,555,256
Less: Share-based compensation cost
130,694
103,329
219,408
178,717
Less: Depreciation
21,070
19,127
42,835
35,972
Less: Amortization of intangibles
—
188
—
375
Adjusted operating expenses
$
746,431
$
695,342
$
1,524,017
$
1,340,192
Reconciliation of GAAP to adjusted income (loss) from operations:
GAAP loss from operations
$
(107,161
)
$
(318,715
)
$
(368,509
)
$
(689,973
)
Plus: Share-based compensation cost
130,694
103,329
219,408
178,717
Plus: Depreciation
23,754
21,307
47,864
40,332
Plus: Amortization of intangibles
1,177
1,028
2,360
2,014
Adjusted income (loss) from operations
$
48,464
$
(193,051
)
$
(98,877
)
$
(468,910
)
View source version on businesswire.com: https://www.businesswire.com/news/home/20240807337131/en/
Permalink
https://www.aetoswire.com/en/news/0708202440767
Contacts
Investor Contact
Liza Heapes
+1 857-302-5663
ir@beigene.com
Media Contact
Kyle Blankenship
+1 667-351-5176
media@beigene.com
No comments:
Post a Comment